Abstract
Introduction: HLA-E is a class I HLA molecule that is involved in NK and CTL functions. It has limited polymorphism but can present unconventional peptides if it is aberrantly expressed under stress conditions. Donor-recipient (D/R) mismatching may potentiate NK-mediated alloreactivity. The functional role of HLA-E in HSCT is uncertain. We used CIBMTR data and samples to investigate the effect of HLA-E polymorphism on HSCT outcome in a 10/10 HLA matched unrelated acute leukemia setting by addressing the following questions: 1) Does D/R HLA-E match status affect HSCT outcome? 2) Does a specific D/R HLA-E genotype correlate with outcome?
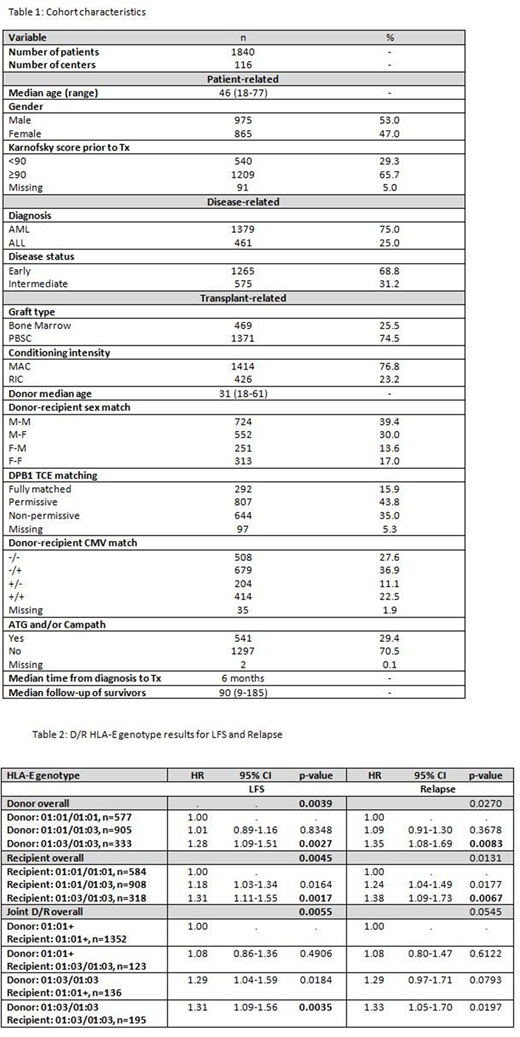
Methods: The study included 1840 adult AML/ALL patients in complete remission who received first T-cell replete, 10/10 high resolution HLA matched transplants from unrelated donors between 2000 to 2015. Cohort characteristics are summarized in Table 1. Both D/R were HLA-E genotyped by NGS using a validated protocol on an Illumina Miseq platform. Overall survival (OS), leukemia free survival (LFS), relapse (RI), transplant related mortality (TRM), acute GvHD (aGvHD) and chronic GvHD (cGvHD) were evaluated; p< 0.01 was considered significant. Factors violating the proportional hazards assumption were adjusted via stratification, while a stepwise model building approach was used to select variables related to a given outcome with a threshold of 0.05 for both entry and retention in the model. HLA-E genotype effects in D/R were tested separately by comparing 0, 1 or 2 copies of a particular HLA-E allele (i.e. HLA-E*01:03). Transplant pairs with HLA-E genotypes including other HLA-E alleles (i.e. other than HLA-E*01:01 or *01:03) were excluded from this analysis. The joint effect of D/R HLA-E genotype was also explored through a 4-level analysis of D/R HLA-E*01:03 homozygous vs other.
Results: HLA-E*01:01 and *01:03 frequencies in both D/R as well as the rate of HLA-E discrepant transplant pairs (32.7%) were in accordance with previously reported data. Distribution of HLA-E matched vs HLA-E mismatched cases was balanced for all significant clinical predictors, while no significant interactions between the HLA-E matching status variable and the adjusted covariates were detected. HLA-E mismatch had no significant association with any of the outcome endpoints in both univariate and multivariate models (data not shown). With respect to the HLA-E genotype effect on outcome, 1827/1840 transplant pairs were considered for analysis, as 13 transplant pairs were excluded due to new or rare HLA-E genotypes. When tested separately, both D/R HLA-E*01:03/01:03 genotypes were associated with significantly lower LFS (HR=1.28, p=0.0027 and HR=1.31, p=0.0017 respectively). While the impact of HLA-E genotype on other outcome endpoints was not statistically significant, relapse rates were higher in cases with D/R HLA-E*01:03/01:03 genotype. Joint D/R HLA-E genotype analysis suggest that donor HLA-E*01:03/01:03 is driving this effect. The results of LFS and RI multivariate models are summarized in Table 2. No significant interactions were observed between the HLA-E genotype variable and the adjusted covariates in the multivariate models.
Conclusion: This is the largest study investigating the effect of HLA-E polymorphism on HSCT outcome. We found no apparent effect of HLA-E mismatch on HSCT outcome in AML/ALL patients in complete remission, while donor HLA-E*01:03/01:03 genotype was associated with lower LFS, contrary to some previous reports. Differential efficiency of the two HLA allelic forms in inducing the CD94/NKG2A inhibitory pathway may account for their divergent effect on leukemia control, although this hypothesis is yet to be confirmed by functional assays. Our results suggest that avoidance of HLA-E*01:03/01:03 donors may improve the outcome of HSCT from 10/10 matched unrelated grafts.
This project was funded by the Else Kröner-Fresenius-Foundation and the National Marrow Donor Program® (NMDP).
Lee:Kadmon: Research Funding; Amgen: Consultancy, Research Funding; Mallinckrodt: Honoraria; Incyte: Consultancy; Pfizer: Consultancy; Onyx: Research Funding; Takeda: Research Funding. Fleischhauer:GENDX: Research Funding. Paczesny:Viracor IBT Laboratories: Patents & Royalties. Schrezenmeier:Alexion Pharmaceuticals, Inc.: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal